WES and RNA-seq analysis of patients treated with menin inhibitor compound SNDX-5613
The following case study features data published from Issa, G.C., Aldoss, I., DiPersio, J. et al. The menin inhibitor revumenib in KMT2A-rearranged or NPM1-mutant leukaemia. Nature 615, 920–924 (2023). https://doi.org/10.1038/s41586-023-05812-3
Study Aim
Syndax Pharmaceuticals recently conducted a phase 1 clinical trial investigating the efficacy of their menin inhibitor compound, revumenib (SNDX-5613). Revumenib is a potent and selective oral inhibitor of the menin–KMT2A interaction in the treatment of patients with relapsed/refractory leukaemia. The trial participants included acute myeloid leukaemia (AML), acute lymphoblastic leukaemia (ALL) and mixed lineage leukaemia (MLL) patients harbouring an MLL gene rearrangement (KMT2Ar) or nucleophosmin 1 (NPM1) mutation.
Up to 90 patients received treatment over a 28-day period, whereby SNDX-5613 was administered twice daily. Samples were collected from patients at various treatment cycles and time points. Whole exome sequencing (WES) data and RNA sequencing (RNA-seq) data were generated from the samples.
Syndax Pharmaceuticals then tasked Fios Genomics with analysing these data sets. The aim of the WES data analysis was to explore somatic co-mutations in patient samples, while the aim of the RNA-seq data analysis was to examine post-dose gene expression changes vs. pre-treatment.
Analysis Results
The RNA-seq analysis identified down-regulation of leukaemogenic target genes (MEIS1, HOXA9, PBX3) and up-regulation of genes associated with haematopoietic cell differentiation (CD11b, CD14) upon administration with menin inhibitor revumenib. The FLT3 gene, a putative transcriptional target of MEIS1, was also down-regulated. The transcriptional changes were consistent with the expected mode of action for a menin inhibitor.
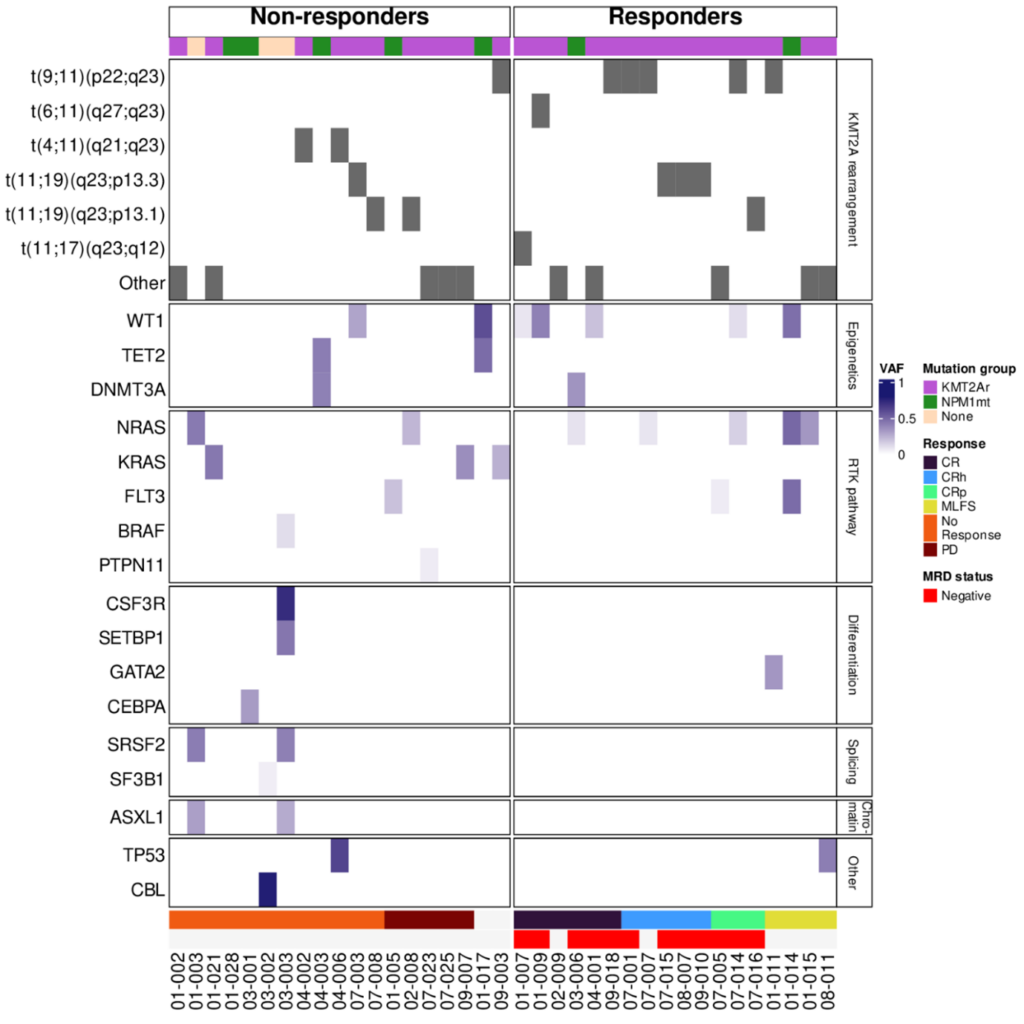
Somatic co-mutation analysis was carried out to explore the mutation profiles of patients. Investigation into somatic mutations and patient response evaluations revealed that responses were not observed in patients that did not harbour either NPM1 mutations or KMT2Ar, consistent with the hypothesis that clinical responses caused by menin inhibition is dependent on the presence of NPM1 mutations or KMT2Ar.
Bioinformatics analysis showed that the transcriptional and genomic landscape of AML, ALL and MLL patients undergoing revumenib treatment followed the expected trajectory of those exposed to a menin inhibitor. Based on analysis of this data set, revumenib may represent an effective therapeutic for refractory acute leukaemia.
Conclusions
There are currently no therapeutics targeted towards leukaemia with NPM1 mutations or KMT2Ar gene rearrangement. Syndax Pharmaceuticals have performed a phase 1 clinical trial which suggests that revumenib may be effective in treating leukaemia with NPM1 mutations or KMT2Ar gene rearrangement.

