Bioinformatics Applications in Biotechnology
- 9th March 2022
- Posted by: Breige McBride
- Category: Bioinformatics
There are various bioinformatics applications in Biotechnology. In this blog, we detail the bioinformatics applications relevant to biotechnology companies which focus on the preclinical phase of the drug discovery and development process.
The Drug Discovery and Development Process
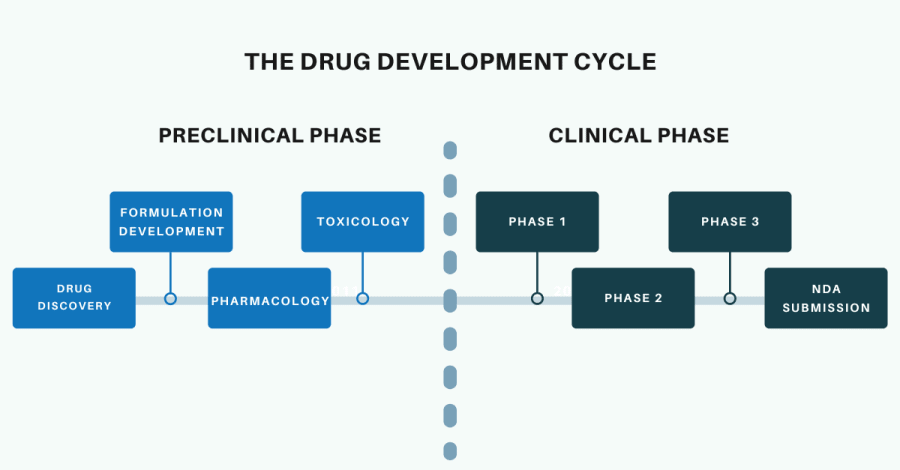
Both the Biotechnology industry and the Pharmaceutical industry focus on the drug discovery and development process. Generally, biotechnology companies concentrate their efforts on the preclinical phase of this process. At some point in the preclinical phase (usually after formal safety and toxicology testing) biotechnology companies will either partner with, or sell their drug candidate to, a pharmaceutical company. The pharmaceutical company will then progress the drug development process into the clinical phase and take on the costs associated with clinical trials.
Bioinformatics Applications in Biotechnology
Bioinformatics approaches can be applied to both the preclinical and clinical stages of the drug discovery and development process. However, since we are discussing bioinformatics applications in biotechnology, we will focus on bioinformatics applications in the preclinical stage. The preclinical stages of the drug discovery and development process are drug discovery, formulation development, pharmacology and toxicology.
Bioinformatics Applications in Drug Discovery
In the drug discovery phase biotech companies work towards rational drug design by researching biological targets. This enables drug design focused on specific biomarkers. Bioinformatics analyses can help biotech companies with biomarker identification, for measurement of drug efficacy and also mechanism of action. Also, bioinformatics approaches can be beneficial earlier in the drug discovery process to assist with drug target and lead identification.
Bioinformatics Applications in Formulation Development
Bioinformatics analyses are useful when comparing the performance of different drug formulations and administration routes. For example, using gene expression analysis we can examine the gene expression changes caused by different formulations. We can also measure the duration of those gene expression changes.
Bioinformatics Applications in Pharmacology
The pharmacology stage of the preclinical drug development cycle focuses on a drug’s safety as well as its ADME. ADME refers to a drug’s Absorption, Distribution, Metabolism and Excretion. Bioinformatics analyses are useful in a number of ways when assessing a drug’s safety and ADME. For example, bioinformatics analyses are often used at this stage to to gain insight into the mechanism of action of a therapeutic and to highlight any immediate safety concerns related to on- or off-target effects. What’s more, we can couple bioinformatics analyses with preclinical pharmacology assays to better inform precision medicine strategies prior to clinical trials, as this webinar shows.
Bioinformatics Applications in Toxicology
When it comes to bioinformatics applications in biotechnology, toxicology is an area where biotech companies can really benefit from bioinformatics expertise. Bioinformatics analyses assist with identifying and validating toxicity response pathways for predictive toxicology. Furthermore, such analyses can provide researchers with context for up- and down-regulated genes – whether the change in regulation could be harmful or whether there are associations that need further investigation.
Bioinformatics Outsourcing for Biotechnology Companies
Biotech companies can utilise bioinformatics to save time throughout the entire preclinical drug discovery and development process. These time savings help Biotechs to reach their end goal, of partnering with or selling their drug asset on to a pharmaceutical company, as quickly and economically as possible.
At Fios Genomics we are renowned not only for our bioinformatics expertise, but also for our speed and quality of work. Also, all of our bioinformaticians have a full understanding of the drug discovery and development process. This enables us to suggest the most suitable bioinformatics analyses to assist with your particular project, no matter what stage of the preclinical process you are at. (We can also provide bioinformatics expertise and analyses to assist with the clinical stages of the drug development process).
To learn more about our bioinformatics services for these areas, visit our preclinical development or clinical development pages. You can also contact us to discuss your particular needs using the form below. We are always happy to discuss potential projects and advise where bioinformatics analyses could be beneficial.
Download RNASeq Data Analysis Report
Fill in the form below to access the data analysis report our team created. Here, bioinformatics analysis of publicly available RNA sequencing (RNASeq) datasets helped to identify genes and pathways associated with psoriasis and the response of psoriatic skin to modulation of the AhR.
At Fios Genomics, we avoid providing clients with lists or more data. Instead, our analysis solutions provide relevant, reliable and actionable information. Our conclusions are data-driven and led by biology rather than statistics, as you can see in our data analysis report examples.
Fill the form below to receive a demo today.
Author: Breige McBride, Content and Social Media Manager, Fios Genomics
Reviewed by Fios Genomics Bioinformatics Experts to ensure accuracy
See Also:
Using Biobank Data To Reach Your Research Goals
Supporting Biomarker Discovery With Bioinformatics
Leave a Reply
You must be logged in to post a comment.

