What is the Drug Development Process? – Clinical Development
- 23rd June 2020
- Posted by: Claudine Gabriele
- Category: Articles
In our last post on drug development, we focussed on the preclinical side of the process, now we will take a look at the clinical drug development process.
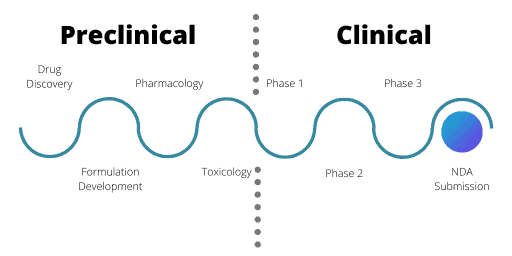
Stages of the Clinical Drug Development Process
The clinical development stages are the first time a drug will be tested in a human. Phase 1 trials are trials with very limited numbers of drugs compared to where the development pipeline started. From thousands of potential candidates in the research and development phases, now only two or three will go forward.
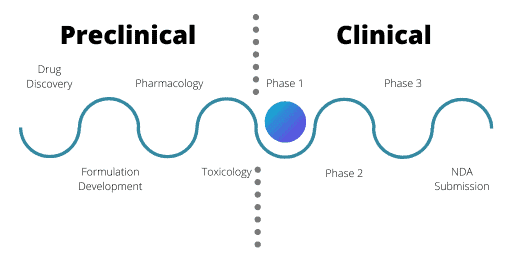
Phase 1
Phase 1 trials centre on a small number of healthy volunteers. Usually, these volunteers are male unless reproductive toxicology tests were conducted during the IND application.
For Phase 1, the aim is to assess safety and PK information. The volunteers receive very small doses of the drug, in ascending amounts through single or multiple doses. The purpose is to evaluate how the human body copes with and gets rid of the drug. This can help find the maximum tolerated dose of the drug as well as flag any acute safety issues. Often Phase 1 trials are very short in length for these reasons.
An exception to using healthy volunteers is for oncology and some specific orphan disease indications. For oncology Phase 1 trials, patients who have exhausted all other forms of treatments can be included as well as advanced cancer patients. Oncology trials may not be for a specific oncology indication – they may be basket trials open to all indications.
Phase 1 trials also assess the efficacy of a drug. However, it is worth noting that patients with the specific disease may respond differently to a drug (when tested in later phase trials) compared with healthy volunteers. For this reason, some trials are not compatible for testing in healthy volunteers as a drug may have a known toxicity level.
The failure rate of a drug in a Phase 1 trial is 30%.
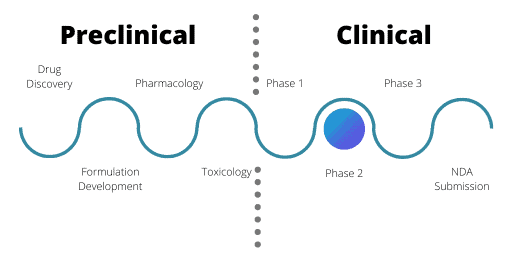
Phase 2 of the Clinical Drug Development Process
Phase 2 trials are the first time that a relevant patient population will a receive a drug, with the exception for oncology and orphan disease therapeutics. Often, trials are split into Phase 2a and 2b. While both have the same overall design, Phase 2a trials usually involve smaller numbers of patients.
Often multiple Phase 2 trials take place in tandem, looking at different clinical indications, combinations or formulations of the drug. To continue from a Phase 2 trial into Phase 3, Phase 2b needs to demonstrate drug efficacy and the trial must meet its primary endpoint.
Overall, Phase 2 trials have a 60% failure rate.
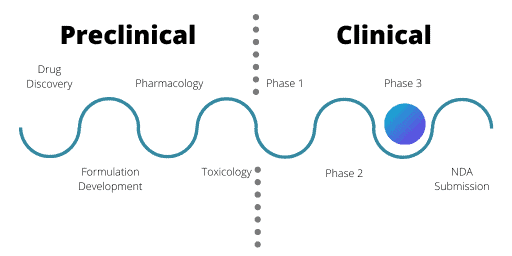
Phase 3
Phase 3 trials are where researchers make comparisons between the new drug and the current standard of treatment. The new drug needs to be more effective than the current drugs on the market or offer other benefits such as easier dosing methods or fewer side effects for patients.
Trials in Phase 3 aim for “real world” drug exposure. They use a wide variety of patient groups, varying drug dose levels and have a greater number of patients. These trials are often very long, with patients potentially receiving treatment over a number of years.
In Phase 3 trials, the failure rate for therapeutics is 30%.
Aside from trials
Other types of testing do not stop while phased trials are ongoing. Reproductive toxicology results are still necessary, if women of child-bearing age will be participate in the trials eventually, or could receive the drug as a patient. Other trials such as longer-term toxicology and carcinogenicity studies are necessary for therapeutics which will potentially be for longer-term use.
The final hurdle – New Drug Application
The final part of the drug development process is the New Drug Application (NDA). This is when a portfolio containing all the drug’s details and trial results is submitted to the relevant regulatory authorities. This includes all preclinical, clinical and other supporting evidence. Strict guidelines, specific to each regulatory agency, must be met.
10% of NDAs fail – usually with further trials necessary to confirm safety or efficacy.
How to reduce the drug development timeline
With a potential lead time of over 15 years for drug development, reducing timelines is vital. Especially in the cases for vaccines of novel drugs, multiple years is often too long of a wait time.
There are many options to reduce the time it takes from development to becoming available for patients. For a new therapeutic, a number of processes, especially early on in development, can run in tandem. Having an understanding of the disease, receiving patient populations and the drug can also help to shorten the time from research and development to patients, as R&D and preclinical development can be more targeted.
Large pharmaceutical companies may also buy out smaller companies with promising pipelines of potential drugs. This helps move the development process along by funding trials, and helps bridge gaps in their portfolio of marketed therapeutics.
Repositioning existing drugs
Repositioning is becoming more common in drug development, as the existing drug has already passed safety tests and a large part of the development process is already complete. In 2007-09, 30-40% of newly approved drugs were either drugs repositioned for new indications, reformulations or new combinations of existing drugs. Repositioning can also extend the patent life on a drug, allowing exclusive marketing for the company which can be vital for recouping costs incurred in the development processes.
Bioinformatics Analyses During the Clinical Drug Development Process
At the clinical stage, analysis and bioinformatics are necessary at all phases, to ensure that trials reach all endpoints, whether primary or secondary. Bioinformatics can also be useful to analyse why a trial has failed, and can help reposition or refocus a drug on a more suitable patient population or disease.
Finally, in repositioning existing drugs, it is possible to save years of time and avoid the significant costs of bringing a new drug to the market. It allows companies to take advantage of their current portfolio, while helping a greater number of patients with their treatments.
Contact us to advance your drug development research with bioinformatics.
See Also:
Fios Genomics on YouTube
The Future of Bioinformatics in 2023
The Clinical Trial Diversity Problem
Leave a Reply
You must be logged in to post a comment.

