Drug Repurposing: What Are The Benefits?
Repurposing or repositioning a drug is where existing, approved drugs are taken and potential new uses for them are identified. This has many benefits for research and treatments, from speed of research to reduction in costs. Drug repurposing is an effective alternative to the standard drug development process that a new molecular entity would have to go through, and can be achieved via both wet lab and computational methods to identify potential new indications.
There are many well-known examples of drugs that were repurposed from their original use. Sildenafil (sold as Viagra), was originally used for cardiovascular treatment and repositioned to treat erectile disfunction. Thalidomide, well-known for its unintended consequences in developing foetuses, was originally developed as a sedative, then repurposed as a nausea drug. More recently, it has been used to treat both leprosy and multiple myeloma. Pembrolizumab (Keytruda) was originally aimed at treating advanced melanoma and has now been repurposed to treat multiple different cancer types.
Drugs may be repurposed for indications that are very different from their original use. Raloxifene was initially developed to treat osteoporosis, but it has since been repositioned to reduce the risk of invasive breast cancer in postmenopausal women. Tamoxifen was originally approved to treat metastatic breast cancers, and is now used to treat bipolar disorder.
During the COVID-19 pandemic, drug repurposing was at the forefront of research into potential treatments. Remdesivir was the first drug to be approved by the FDA for treatment use. It was originally used to treat hepatitis C and respiratory syncytial virus (RSV), and also had previous history of being repurposed – during the 2014-2016 outbreak of Ebola in West Africa, although it ultimately proved ineffective for that indication.
Benefits of drug repurposing
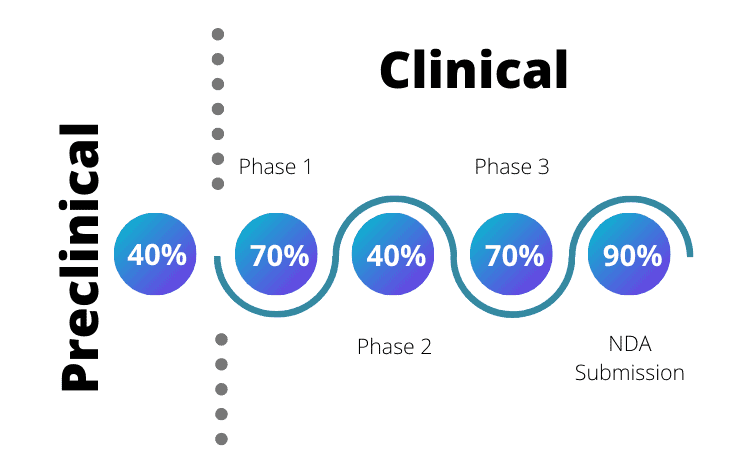
Each number in the image above highlights the percentage of drugs that pass through to the next stage of drug development and finally into the marketplace.
As you can see, over 60% of potential new drugs fail at the preclinical stage. Once a molecule moves into clinical testing, there is still a high attrition rate at each phase. One of the key appeals of drug repurposing is the fact that the drug has already been through preclinical and clinical trials and has cleared those stages. While care does still need to be taken, such as if a drug is to be used on a group that was not present in the original safety trials (as was the case for thalidomide), the underlying safety in humans has already been proven in previous trials.
These drugs are essentially ‘de-risked’ to a large extent, as safety data is already a known entity for the company. This in turn has beneficial knock-on effects on costs and development timelines. Without the need to conduct preclinical trials, a typical 15-year development timeline is radically shortened, and costs along with it.
For companies, drug repurposing also has another benefit in that it can extend the patent life of a drug. When the cost of creating and moving a drug to market is between £350M and £2B, and only 4% of all drugs developed make it into the marketplace and return their investment, the ability to reposition a drug to gain more years of exclusive marketing can be vital to recoup costs.
Fios and drug repurposing
Repositioning drugs can cross therapeutic research areas. At Fios, we have a diverse range of expertise covering many different research areas, ensuring we can support your research no matter the therapeutic area you aim to target.
Additionally, we can work to help repurpose your therapeutics through wet lab and in silico analysis. We can analyse both on- and off-target effects to ensure you understand the impact your molecules have on potential therapeutic indications, as well as mine publicly available datasets for markers in potential indications of interest relevant for your repositioning.
Have a drug repurposing project you would like to discuss? Contact us today.
Read more
Drug repurposing: progress, challenges and recommendations (2018)
Drug Repurposing (DR): An Emerging Approach in Drug Discovery (2020)
What is the Drug Development Process? – Clinical Development (2020)
An Overview of the Drug Development Process – Preclinical Stage (2020)

