Bioinformatics and the Pharmaceutical Industry
- 25th October 2021
- Posted by: Breige McBride
- Category: Bioinformatics
The pharmaceutical industry uses bioinformatics throughout the drug discovery and development process. In this blog we explain which particular analyses are beneficial at each stage.
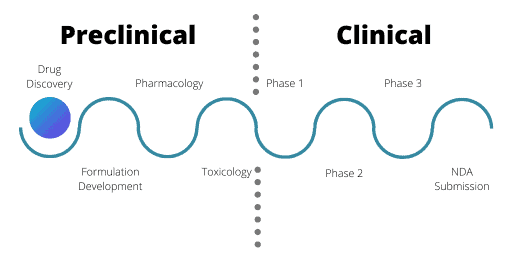 Stages of the Drug Discovery and Development Process
Stages of the Drug Discovery and Development Process
Preclinical Stage
Identifying Drug Targets and Leads
The overall purpose of pharmaceutical companies is to create new drugs to treat diseases and other medical conditions. In order to create a new drug, we first need to identify its drug target. This is a molecule in the body (such as a protein or gene) that is associated with the disease to be treated. Pharmaceutical companies also need to develop a chemical compound that has the potential to treat the disease. Researchers refer to this as a drug lead until they know it is safe to use and successful at treating the disease.
How Bioinformatics Helps the Pharmaceutical Industry Identify Drug Targets and Leads
Bioinformatics approaches help identify drug targets and leads in a variety of ways. For example, analyses of the data generated from High-Throughput Screens (HTS) can be used to identify a drug lead. Meanwhile, analyses of data from preclinical experiments using animal models, diseased human tissues and cell lines can identify drug targets as well as leads.
Validation of Drug Targets and Leads
Once they have been identified, drug targets and leads then need to be validated. To do this you must demonstrate that the drug lead affects the target molecule in a way that provides a positive therapeutic outcome.
Using Bioinformatics to Validate Drug Targets and Leads
Target validation can be supported by robust analyses of CRISPR screen data or gene expression data generated from models in which the target has been knocked-out or knocked down.
Lead validation can be established by comparing gene expression data from untreated models and models treated with the identified lead. This comparison gives insights into the lead mode of action.
Additionally, both drug target validation and drug lead validation can be supported by in silico comparisons of preclinical results with publicly available data.
Clinical Stage
The clinical stage in drug development is when researchers administer the drug to human volunteers in clinical trials. There are 3 phases of clinical trials and each has a different aim. The primary aim of Phase 1 trials is to test the safety of a drug in healthy volunteers. Phase 1 trials also assess what dose of the drug the human body can tolerate and may assess an element of drug efficacy.
Phase 2 trials then test the drug in the relevant patient population (exceptions are oncology drugs and orphan disease therapeutics). Often there are 2 parts of Phase 2 trials; Phase 2a and Phase 2b. In these cases, Phase 2a trials test a smaller number of patients. It is common for multiple Phase 2 trials to run simultaneously, testing various clinical indications, combinations, or formulations of the drug.
Finally, there are Phase 3 trials. In these trials, researchers compare the trial drug to current drugs already available to treat the same condition. To pass a Phase 3 trial the trial drug must be better than the current drugs available. For example, the new drug must be more effective or have less side effects.
How Bioinformatics Benefits the Pharmaceutical Industry at the Clinical Stage
Phase 1, 2, and 3 trials all generate data, often in vast amounts. This is where bioinformatics comes in. Bioinformatics analyses can be applied to data from primary outcomes as well as each of the following data types which can be generated during clinical trials:
- Genotype Data
- Demographic Data
- Gene Expression Data
- Clinical Phenotypes
- Clinical Chemistry
- Immunohistochemistry (IHC)
- Flow Cytometry
- Haematological tests
- Human leukocyte antigen (HLA) typing
- Cytokine profiling
- Pharmacokinetic/pharmacodynamic measurements
Applying bioinformatics approaches to clinical trial data ensures we reveal all of the key insights in the data. In turn, these insights can then inform the next steps and ensure maximum return on investment in pharmaceutical research.
What’s more, we can use bioinformatics approaches to integrate multiple data types. In fact, almost all types of biological data can be integrated together for analysis. For example, in preclinical development we can combine gene expression changes with copy number variant (CNV) and mutational data to identify biomarkers that are indicative of a compound’s sensitivity. Meanwhile, in clinical development, we can integrate different data types to help assess safety, tolerability, and efficacy for patients. For example, we can combine proteomics data with pharmacokinetics (PK) data to assess patient outcomes after treatment and relate response to exposure.
Drug Repurposing
Bioinformatics providers can also support the pharmaceutical industry when it comes to drug repurposing. Bioinformatics services can help repurpose drugs via:
- Signature generation of compound effects
- In silico assessment of potential indications for repurposing using on- and off-target effects
- Data landscaping and mining for markers in potential indications of interest
These services help pharmaceutical companies to maximise the value of their drug pipelines by repurposing or repositioning their drug or novel therapeutic for new indications.
Summary
As explained above, bioinformatics is an essential tool in the pharmaceutical industry’s toolbox. Bioinformatics analyses provide key information throughout the entire drug discovery and development process, from aiding the identification and validation of drug targets and leads through to helping assess the outcomes of phase 1, 2 and 3 clinical trials; as well as supporting drug repurposing efforts.
Pharmaceutical companies regularly rely on Fios Genomics’ bioinformatics services to get the most out of their research data. You can view the full range of services we provide on our bioinformatics services page. If you would like to learn more about bioinformatics support for your drug discovery or development project, then please contact us.
Or, to learn more about what it is like working with Fios Genomics, please visit our clients and partners page.
Thanks for visiting our blog!
Author: Breige McBride, Content and Social Media Manager, Fios Genomics
Reviewed by Fios Genomics Bioinformatics Experts to ensure accuracy
See also:
Using Biobank Data to Reach Your Research Goals
How ‘omics analysis can better inform precision medicine strategies prior to clinical trials
Overview of the Preclinical Phase of the Drug Development Process
Overview of the Clinical Phase of the Drug Development Process
Download RNASeq Data Analysis Report
Fill in the form below to access the data analysis report our team created. Here, bioinformatics analysis of publicly available RNA sequencing (RNASeq) datasets helped to identify genes and pathways associated with psoriasis and the response of psoriatic skin to modulation of the AhR.
Leave a Reply
You must be logged in to post a comment.

